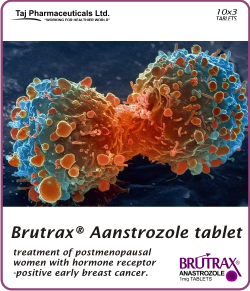
BRUTRAX®
Anastrozole 1mg Tablets
 |
 |
 |
 |
Contact us |
Address: |
|
BRUTRAX® (anastrozole) Tablets BRUTRAX® (anastrozole 1mg Tablets)Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international double-blind randomised placebo-controlled trial Anastrozole is used to treat breast cancer in women who have already gone through menopause and no longer have menstrual periods (postmenopausal women ) Anastrozole (INN) (marketed under the trade name Brutrax by AstraZeneca) is a non-steroidal aromatase-inhibiting drug approved for treatment of breast Consumer information about the medication ANASTROZOLE - ORAL (Brutrax) includes side effects drug interactions recommended dosages and storage Official Web site for BRUTRAX® (anastrozole) Tablets Learn about an early breast cancer treatment option breast cancer treatment coping strategies nutrition Learn about the prescription medication Brutrax (Anastrozole) Get information about a hormonal therapy called Anastrozole (Brutrax ®) which is used to treat breast cancer Physician reviewed anastrozole patient information - includes anastrozole description dosage and directions Anastrozole is used to treat breast cancer in women who have already gone through menopause and no longer have menstrual periods Anastrozole Sandoz is a non-steroidal aromatase inhibitor which reduces the amount of oestrogen (female sex hormone) made by the body in postmenopausal Anastrozole medical facts from brutrax com A new way to prevent breast cancer: anastrozole - Cancer Research BRUTRAX anastrozole 1 mg breast cancer treatment coping nutrition exercise community support side effects dosage prescribing information anastrozole oral Brutrax anastrozole oral effectiveness satisfaction ease of use medication medications medicine drug drugs prescription drugs user ratings drug ratings drug reviews rate a drug treatment side effects drug interactions drug information medical information medical advice warnings overdose drug images over the counter indications precautions Brutrax Anastrozole drug description chemical structure black box warning medications drugs generic fda approved patient labeling reviews professionals clinicians clinical anastrozole directions dose anastrozole Brutrax breast cancer aromatase inhibitor hormones hormone treatment side effects therapy hormonal Brutrax hormone antagonist aromatase inhibitor postmenopausal breast anastrozole tablets manufacturer anastrozole tablets supplier anastrozole export import anastrozole Generic anastrozole tablets manufacturer anastrozole tablets anastrozole tablets price manufacturing possibilities medicine health uses anastrozole trials anastrozole manufacturing anastrozole India trading anastrozole product anastrozole supplier supple anastrozole suppliers anastrozole vendor anastrozole vendors price anastrozole prices
|
Full Prescribing Information
CLINICAL PHARMACOLOGY
Mechanism of Action
The growth of many cancers of the breast is stimulated or maintained by estrogens.
In postmenopausal women, estrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens (primarily androstenedione and testosterone) to estrone and estradiol. The suppression of estrogen biosynthesis in peripheral tissues and in the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Anastrozole is a selective non-steroidal aromatase inhibitor. It significantly lowers serum estradiol concentrations and has no detectable effect on formation of adrenal corticosteroids or aldosterone.
Pharmacodynamics
Effect on Estradiol
The effect of anastrozole in premenopausal women with early or advanced breast cancer has not been studied. Because aromatization of adrenal androgens is not a significant source of estradiol in premenopausal women, anastrozole would not be expected to lower estradiol levels in premenopausal women.
Effect on Corticosteroids
In multiple daily dosing trials with 3, 5, and 10 mg, the selectivity of anastrozole was assessed by examining effects on corticosteroid synthesis. For all doses, anastrozole did not affect cortisol or aldosterone secretion at baseline or in response to ACTH. No glucocorticoid or mineralocorticoid replacement therapy is necessary with anastrozole.
Pharmacokinetics
Absorption
Absorption of anastrozole is rapid and maximum plasma concentrations typically occur within 2 hours of dosing under fasted conditions. Studies with radiolabeled drug have demonstrated that orally administered anastrozole is well absorbed into the systemic circulation. Food reduces the rate but not the overall extent of anastrozole absorption.
Distribution
Steady-state plasma levels are approximately 3- to 4-fold higher than levels observed after a single dose of anastrozole. Plasma concentrations approach steady-state levels at about 7 days of once daily dosing. Anastrozole is 40% bound to plasma proteins in the therapeutic range.
Metabolism
Metabolism of anastrozole occurs by N-dealkylation, hydroxylation and glucuronidation. Three metabolites of anastrozole (triazole, a glucuronide conjugate of hydroxy-anastrozole, and a glucuronide conjugate of anastrozole itself) have been identified in human plasma and urine. The major circulating metabolite of anastrozole, triazole, lacks pharmacologic activity.
Excretion
Eighty-five percent of radiolabeled anastrozole was recovered in feces and urine. Hepatic metabolism accounts for approximately 85% of anastrozole elimination. Renal elimination accounts for approximately 10% of total clearance. The mean elimination half-life of anastrozole is 50 hours.
Effect of Gender and Age / Race
Anastrozole pharmacokinetics have been investigated in postmenopausal female volunteers and patients with breast cancer. No age-related effects were seen over the range <50 to >80 years.
Estradiol and estrone sulfate serum levels were similar between Japanese and Caucasian postmenopausal women who received 1 mg of anastrozole daily for 16 days.
Effect of Renal Impairment / Hepatic Impairment
Anastrozole pharmacokinetics have been investigated in subjects with renal impairment.
Anastrozole pharmacokinetics have been investigated in subjects with hepatic cirrhosis related to alcohol abuse.
VISION
 At Taj Pharmaceuticals our goal is to create innovative drugs and diagnostic tools for the medicine of tomorrow. Rapid progress in the sciences is providing crucial insights into the underlying molecular mechanisms of disease, opening up new ways for prevention, diagnosis and therapy. With the expertise in Diagnostics and Pharmaceuticals, Taj Pharmaceuticals is best placed to capture the value from the Healthcare Revolution.
At Taj Pharmaceuticals our goal is to create innovative drugs and diagnostic tools for the medicine of tomorrow. Rapid progress in the sciences is providing crucial insights into the underlying molecular mechanisms of disease, opening up new ways for prevention, diagnosis and therapy. With the expertise in Diagnostics and Pharmaceuticals, Taj Pharmaceuticals is best placed to capture the value from the Healthcare Revolution.
The future will be shaped by fundamental paradigm shifts ushering in a new era of more effective medicine. Diagnosis, prevention and treatment will be linked more closely than ever before in integrated strategies offering patients and healthcare providers highly focused, individualised care. We are confident that, with our outstanding workforce, cutting-edge science and state-of-the-art technology base, we can meet tomorrow's needs for integrated healthcare solutions.
MISSION

Taj Pharmaceuticals is committed to a system of corporate governance that conforms to the latest standards and takes due account of our corporate development. The safety and health of our employees and the public, as well as the protection of our neighbourhoods and the environment, have high priority at all Group facilities. In all we do, we are guided by the principle of sustainable development.
Download Brutrax Overview
Brutrax® (Aastrozole Tablet) may help reduce your risk of breast cancer recurrence sold by Taj Pharmaceuticals Limited (India), a global pharmaceuticals company. It is available in India, Middle East and a few other South Asian countries.
![]() Patient Counseling Information PDF
Patient Counseling Information PDF
![]() Full Prescribing Information PDF
Full Prescribing Information PDF
Note: This product information is intended only for residents of the India. Taj Pharmaceuticals Limited, medicines help to treat and prevent a range of conditions—from the most common to the most challenging—for people around the world. The Price of the drugs indicated above may not match the actual price at which they are sold. Prices can change depending on many factors, including local taxes. These are only approximate indicative prices of the drug. The products discussed herein may have different product labelling in different countries. The product information provided in this site is intended only for the residents of India.
Information for Health Care Professionals
*** Please consult local Prescribing Information for any product before use. This website is an international information resource for healthcare professionals with an interest in disease management. This website is not intended to replace the advice of a qualified healthcare professional. Above brand is a trademark of the Taj group of companies (Taj Pharmaceuticals Limited).
Taj Pharma Group
TajPharmaceuticals India

Pharmaceuticals Manufacturing Company Taj API

Active Pharmaceuticals Ingredients Taj Agro

FMCG Company India Taj Drugs

Generics Pharmaceuticasls Exporter Taj Lifesciences

Lifesciences Company Taj Hospitals

Hospitals in India Taj For Doctors

Information to Patients and Doctors




 Anastrozole 1mg Tablets.png)